Scientists Achieve Breakthrough Synthesis of Naphthocyclinones After Decades
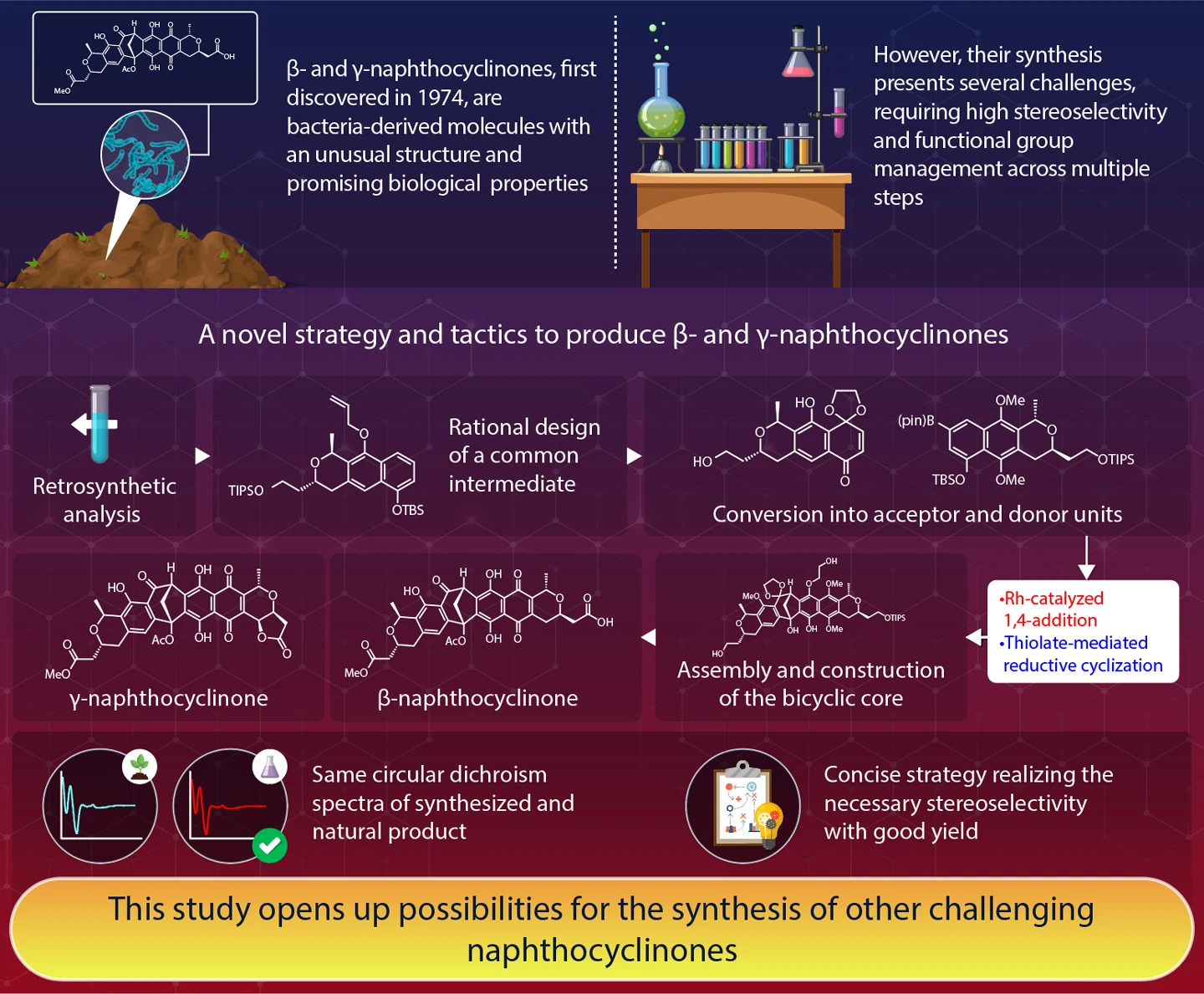
In a groundbreaking achievement, **scientists from Science Tokyo have successfully synthesized β- and γ-naphthocyclinones**. These complex molecules, first discovered in 1974 as red pigments in soil bacteria, represent a family of antibiotics with potential medical benefits. For decades, their synthesis has been a challenge due to the intricate bicyclo[3.2.1]octadienone core structure required to connect monomers A and B. The researchers, led by Associate Professor Yoshio Ando, utilized **an innovative retrosynthetic analysis** approach, working backwards from the target molecules. They crafted a two-step synthesis involving Rh-catalyzed 1,4-addition and thiolate-mediated reductive cyclization, streamlined by designing a common intermediate for both monomers. Their strategy yielded β-naphthocyclinone at a 70% yield for each reaction step. Subsequently, γ-naphthocyclinone was synthesized from β-naphthocyclinone through oxidative lactonization, achieving an impressive 87% yield. This study, published in **Angewandte Chemie International Edition**, provides a roadmap to naphthocyclinone synthesis and opens avenues for the creation of other complex molecules with significant scientific potential.